No other subject in college annoyed me more than organic chemistry. As a biology and geology student, organic chem was a necessary evil and while I enjoyed certain aspects of the subject, like the labs, nucleic acids and such, most of organic chemistry was alphabet soup. I’d trade double doses of any subject, inorganic chemistry, physics, German, or religion, to skip the whole organic thing. But . . . there was one aspect of organic chemistry I loved and was good at . . . sugar chemistry! I loved those little sugar molecules as much as dogs like rolling in . . . well, you get the idea. I could just throw myself into a big pile of those wonderful carbohydrates, academically speaking, be it glucose, fructose, maltose, lactose, or galactose. Love them all! And the best part? Chemistry majors hate sugar chemistry! Too much memorization, they complain. Aw, poor little brain dead chemistry majors!
Another aspect I enjoyed about organic chemistry was the lectures. Not that I’m crazy about sitting in a lecture hall, but the instructor for this class was entertaining as hell and a hell of a nice guy, too. His name was Dr. Ned Heindel, who stood at the bottom of a steeply-tiered lecture room in Chandler Lab, puffing his cigar and scribbling madly on a set of black boards that stretched across the front of the room. He entered the lecture hall one morning and announced he would teach one of my favorites– sugar isomers and stereoisomerism! Now, if you’re chemically challenged, you may ask, “What the hell is an isomer?” Simply put, it’s chemical compounds whose molecular formula is the same, but their structural formula, or arrangement, is different. You might say one of your pants pockets contains the same number and denomination of bills as the other, but you’re spending it on different things. Stereoisomers, on the other hand, are compounds with the same molecular formula, the same structural formula, but are oriented, or turned, a little differently in three-dimensional space. Without throwing in the definition of enantiomers, dextrorotatory sucrose, levorotatory sucrose, and the gauche effect, suffice it to say that sugar molecules suffer from a form of stereoisomerism that, in layman’s terms, means they are mirror images of each other. Behold the tasty little glucose molecules below:
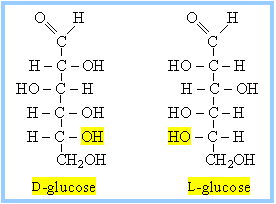 It’s like looking into a mirror, that is if you’re a sugar molecule. A good macro-demonstration is to place both hands, yes, your hands, palms down on a table or desk in front of you. Although your hands are essentially the same, they’re now backwards of each other or mirror images of each other. That’s stereoisomerism in a nut shell!
It’s like looking into a mirror, that is if you’re a sugar molecule. A good macro-demonstration is to place both hands, yes, your hands, palms down on a table or desk in front of you. Although your hands are essentially the same, they’re now backwards of each other or mirror images of each other. That’s stereoisomerism in a nut shell!
This fateful morning in the lecture hall, someone else was way ahead of poor, unsuspecting Dr. Heindel. This mystery person knew the morning’s topic, knew stereoisomerism, and wasn’t afraid to use it. The trap was wisely concealed behind a series of vertical sliding chalkboards that were in the center of the front wall. As the naive professor lectured and scribbled on the blackboard, he heaved each 3′ x 4′ slate upward in a cloud of chalk dust revealing a clean fresh slate beneath. He exhausted a second slate with chalk-scrawled carbon chains and handsome enantiomers, then stuck his cigar back into his mouth and grabbed the handle at the bottom of the blackboard. He heaved it upward to expose the third slate.
 WARNING: The following is rated PG-13. Parental discretion is advised! Click on the chemistry blackboard on the right to see what Dr. Heindel, and his class, witnessed that fateful morning in the lecture hall . . .
WARNING: The following is rated PG-13. Parental discretion is advised! Click on the chemistry blackboard on the right to see what Dr. Heindel, and his class, witnessed that fateful morning in the lecture hall . . .
